Geographic Summary
La Parguera
Puerto Rico
Summary information for the La Parguera site is a compilation of existing
sources, selected documents, and Internet accessible data, which are referenced
by section. The Geographic Summary is intended to provide a brief synopsis
concentrating on coral reef ecosystem, coastline vegetation, and mangroves. It
is not meant to be an in-depth treatise on the geography and background of the
area.
Additional statements that further clarify issues are from multiple sources
including Open File documents, government documents, Internet accessible data
and are listed under Resources Consulted found at the end of the geographic
summary. Only direct quotes or facts are cited. General information from
multiple sources and data from Internet websites are not specifically cited.
Several articles are abridged with original cited sources.
La
Parguera, Puerto Rico
The Geography of Puerto Rico
Puerto Rico is a territory of the United States with commonwealth status. The
island of Puerto Rico is located between the Caribbean Sea and the North
Atlantic Ocean, east of the Dominican Republic and approximately 1000 miles
east-southeast of Miami, Florida. Puerto Rico is an important location along the
Mona Passage, which is a key shipping lane to the Panama Canal. The City of San
Juan has one of the largest and best natural harbors in the Caribbean.

Source: CIA World Factbook: https://www.cia.gov/library/publications/the-world-factbook/geos/rq.html
The La Parguera site is located on the southwestern edge of
Puerto Rico. According to Guild et.al.(n.d), “[The] La Parguera [shelf] has
numerous bank reefs that protect the shore from intense wave action, resulting
in extensive seagrass meadows and a coastline dominated by mangroves with algal
plains, sandy lagoons, and two bioluminescent bays.”
Note: Build up of coastal areas has resulted in sewage
outfalls at La Parguera; overfishing and changes in biodiversity leads to rapid
coral die-back.
Source: Guild, Liane, B. Lobitz J. Goodman, R.
Armstrong, F. Gilbes, R. Berthold, and J. Kerr. n.d. Imaging
spectroscopy and spectral analysis in support of coral reef ecosystem
biodiversity research,
Geography and Coral Reefs
Source:
USGS, Northern Prairie Pine Research Center (http://www.npwrc.usgs.gov/resource/wetlands/classwet/estuarin.htm)
Reef
Definition.
The Class Reef includes ridge-like or mound-like structures formed by the
colonization and growth of sedentary invertebrates. Water regimes are
restricted to subtidal, irregularly exposed, regularly flooded, and
irregularly flooded.
Description.
Reefs are characterized by their elevation above the surrounding substrate
and their interference with normal wave flow; they are primarily subtidal,
but parts of some reefs may be intertidal as well. Although corals, oysters,
and tube worms are the most visible organisms and are mainly responsible for
reef formation, other mollusks, foraminifera, coralline algae, and other
forms of life also contribute substantially to reef growth. Frequently,
reefs contain far more dead skeletal material and shell fragments than
living matter.
Subclasses and Dominance Types.
Coral Reef Structure and Development
Information on the Structure of Coral reefs is condensed from the
original version available at: NOAA’s Coral Reef Information System (CoRis):
http://coris.noaa.gov/about/what_are
Photographs were omitted from the geographic summary
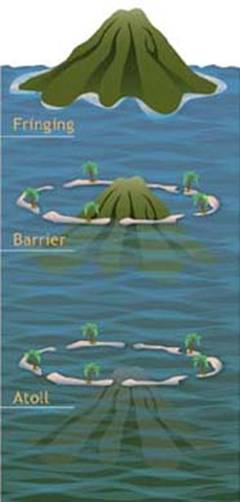 |
Darwin’s
three stages of atoll formation. Coral reefs begin to form when free-swimming coral larvae (planulae) attach to the submerged edges of islands or continents. As the corals grow and expand, reefs take on one of three major characteristic structures—fringing, barrier or atoll.Fringing reefs, which are the most common, project seaward directly from the shore, forming borders along the shoreline and surrounding islands. Barrier reefs also border shorelines, but at a greater distance. They are separated from their adjacent land mass by a lagoon of open, often deep water. If a fringing reef forms around a volcanic island that subsides completely below sea level while the coral continues to grow upward, an atoll forms. Atolls are usually circular or oval, with a central lagoon. Parts of the reef platform may emerge as one or more islands, and breaks in the reef provide access to the central lagoon (Lalli and Parsons, 1995; Levinton, 1995; Sumich, 1996). In the 1830s, Charles Darwin distinguished
between the three main geomorphological categories of reefs, and suggested that
fringing reefs, barrier reefs, and atolls were all related stages in the
sequence of atoll reef formation. All three reef types—fringing, barrier and
atoll—share similarities in their biogeographic profiles. Bottom topography,
depth, wave and current strength, light, temperature, and suspended sediments
all act to create characteristic horizontal and vertical zones of corals, algae
and other species. While these zones vary according to the location and type of
reef, the major divisions common to most reefs, as they move seaward from the
shore, are the reef flat, reef crest or algal ridge, buttress zone, and seaward
slope. |
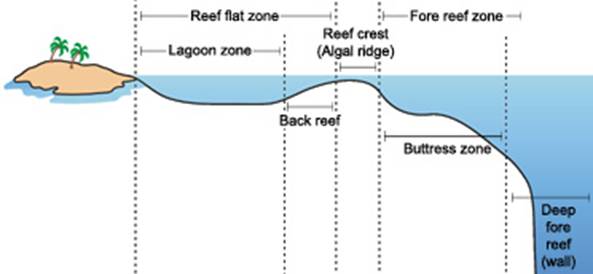
Graphic of typical coral reef zones.
The reef flat, or back reef, is
located on the sheltered side of the reef. It extends outward from the shore;
and may be highly variable in character. Varying in width from 20 or 30 meters
to more than a few thousand, the reef flat may range from only a few centimeters
to a few meters deep, and large parts may be exposed at low tide. The substrate
is formed of coral rock and loose sand. Beds of sea grasses often develop in the
sandy regions, and both encrusting and filamentous algae are common.
Because it is so shallow, this
area experiences the widest variations in temperature and salinity, but it is
protected from the full force of breaking waves. Reduced water circulation, the
accumulation of sediments, and periods of tidal emersions—when the reef is
exposed during low tide—combine to limit coral growth. Although living corals
may be scarce except near the seaward section of this zone, its many
microhabitats support the greatest number of species in the reef ecosystem, with
mollusks, worms and decapod crustaceans often dominating the visible macrofauna
(Barnes, R.D., 1987; Lalli and Parsons, 1995; Sumich, 1996).
The reef crest, or algal ridge,
is the highest point of the reef, and is exposed at low tide. Lying on the outer
side of the reef, it is exposed to the full fury of incoming waves. The width of
this zone typically varies from a few, to perhaps 50 m. In this severe habitat,
a few species of encrusting calcareous red algae flourish, producing new reef
material as rapidly as the waves erode it. Where wave action is severe, living
corals are practically nonexistent, but in situations of more moderate wave
action, the reef crest tends to be dominated by stoutly branching corals. These
closely growing, robust colonies form ramparts able to withstand the heavy seas.
Small crabs, shrimps, cowries and other animals reside in the labyrinthine
subsurface cavities of the reef crest, protected from waves and predators
(Barnes, R.D., 1987; Lalli and Parsons, 1995; Sumich, 1996).
The outermost seaward slope
(also called the fore-reef) extends from the low-tide mark into deep water. Just
below the low-tide mark to approximately 20 m depth is a rugged zone of spurs,
or buttresses, radiating out from the reef. Deep channels that slope down the
reef face are interspersed between the buttresses. These alternating spurs and
channels may be several meters wide and up to 300 m long (Barnes, R.D. 1987;
Lalli and Parsons, 1995; Sumich, 1996).
The buttress zone serves two
main purposes in the reef system. First, it acts to dissipate the tremendous
force of unabating waves and stabilizes the reef structure. Second, the channels
between the buttresses drain debris and sediment off the reef and into deeper
water. Massive corals and encrusting coralline algae thrive in this zone of
breaking waves, intense sunlight, and abundant oxygen. Small fish inhabit the
many holes and crevices on this portion of the reef, and many larger fish
including sharks, jacks, barracudas and tunas patrol the buttresses and grooves
in search of food (Barnes, R.D., 1987; Lalli and Parsons, 1995; Sumich, 1996).
Continuing down the seaward
slope to about 20 m, optimal light intensity decreases, but reduced wave action
allows the maximum number of coral species to develop. Beginning at
approximately 30 to 40 m, sediments accumulate on the gentle slope, and corals
become patchy in distribution. Sponges, sea whips, sea fans, and ahermatypic
(non-reef-building) corals become increasingly abundant and gradually replace
hermatypic corals in deeper, darker water (Barnes, R.D., 1987; Lalli and
Parsons, 1995; Sumich, 1996).
Massive reef structures are
formed when each stony coral polyp secretes a skeleton of CaCO3. Most
stony corals have very small polyps, averaging 1 to 3 mm in diameter, but entire
colonies can grow very large and weigh several tons. Although all corals secrete
CaCO3, not all are reef builders. Some corals, such as Fungia sp.,
are solitary and have single polyps that can grow as large as 25 cm in diameter.
Other coral species are incapable of producing sufficient quantities of CaCO3
to form reefs. Many of these corals do not rely on the algal metabolites
produced by zooxanthellae, and live in deeper and/or colder waters beyond the
geographic range of most reef systems (Barnes, R.D., 1987; Sumich, 1996).
The skeletons of stony corals
are secreted by the lower portion of the polyp. This process produces a cup,
called the calyx, in which the polyp sits. The walls surrounding the cup are
called the theca, and the floor is called the basal plate. Thin, calcareous
septa (sclerosepta), which provide structural integrity, protection, and an
increased surface area for the polyp’s soft tissues, extend upward from the
basal plate and radiate outward from its center. Periodically, a polyp will lift
off its base and secrete a new floor to its cup, forming a new basal plate above
the old one. This creates a minute chamber in the skeleton. While the colony is
alive, CaCO3 is deposited, adding partitions and elevating the coral.
When polyps are physically stressed, they contract into the calyx so that
virtually no part is exposed above the skeletal platform. This protects the
organism from predators and the elements (Barnes, R.D., 1987; Sumich, 1996).
At other times, the polyp
extends out of the calyx. The timing and extent to which a polyp extends from
its protective skeleton often depends on the time of the day, as well as the
species of coral. Most polyps extend themselves furthest when they feed on
plankton at night.
In addition to a substantial
horizontal component, the polyps of colonial corals are connected laterally to
their neighbors by a thin horizontal sheet of tissue called the coenosarc, which
covers the limestone between the calyxes. Together, polyps and coenosarc
constitute a thin layer of living tissue over the block of limestone they have
secreted. Thus, the living colony lies entirely above the skeleton (Barnes,
R.S.K. and Hughes, 1999).
Colonies of reef-building (hermatypic)
corals exhibit a wide range of shapes, but most can be classified within ten
general forms. Branching corals have branches that also have (secondary)
branches. Digitate corals look like fingers or clumps of cigars and have no
secondary branches. Table corals are table-like structures of fused branches.
Elkhorn coral has large, flattened branches. Foliose corals have broad
plate-like portions rising above the substrate. Encrusting corals grow as a thin
layer against the substrate. Submassive corals have knobs, columns or wedges
protruding from an encrusting base. Massive corals are ball-shaped or
boulder-like corals which may be small as an egg or large as a house. Mushroom
corals resemble the attached or unattached tops of mushrooms. Cup corals look
like egg cups or cups that have been squashed, elongated or twisted (McManus et
al. 1997). While the growth patterns of stony coral colonies are primarily
species-specific, a colony’s geographic location, environmental factors (e.g.,
wave action, temperature, light exposure), and the density of surrounding corals
may affect and/or alter the shape of the colony as it grows (Barnes, R.D. 1987;
Barnes, R.S.K. and Hughes 1999, Lalli and Parsons, 1995).
In addition to affecting the
shape of a colony’s growth, environmental factors influence the rates at which
various species of corals grow. One of the most significant factors is sunlight.
On sunny days, the calcification rates of corals can be twice as fast as on
cloudy days (Barnes, R.S.K. and Hughes, 1999). This is likely a function of the
symbiotic zooxanthellae algae, which play a unique role in enhancing the corals’
ability to synthesize calcium carbonate. Experiments have shown that rates of
calcification slow significantly when zooxanthellae are removed from corals, or
when corals are kept in shade or darkness (Lalli and Parsons 1995).
In general, massive corals tend
to grow slowly, increasing in size from 0.5 cm to 2 cm per year. However, under
favorable conditions (high light exposure, consistent temperature, moderate wave
action), some species can grow as much as 4.5 cm per year. In contrast to the
massive species, branching colonies tend to grow much faster. Under favorable
conditions, these colonies can grow vertically by as much as 10 cm per year.
This fast growth rate is not as advantageous as it may seem, however. Mechanical
constraints limit the maximum size that branching corals can achieve. As they
become larger, a heavier load is placed on the relatively small area attached to
the substratum, rendering the colony increasingly unstable. Under these
circumstances, the branches are prone to snapping off during strong wave action.
The opposite is true of the massive-shaped corals, which become more stable as
they grow larger (Barnes, R.S.K. and Hughes, 1999).
Barnes, R.D. 1987. Invertebrate
Zoology; Fifth Edition. Fort Worth, TX: Harcourt Brace Jovanovich College
Publishers. pp. 92-96, 127-134, 149-162.
Barnes, R.S.K. and R.N. Hughes.
1999. An Introduction to Marine Ecology; third edition. Oxford, UK:
Blackwell Science Ltd. pp. 117-141.
Lalli, C.M. and T.R. Parsons. 1995.
Biological Oceanography: An Introduction. Oxford, UK:
Butterworth-Heinemann Ltd. pp. 220-233.
Levinton, J.S. 1995. Marine
Biology: Function, Biodiversity, Ecology. New York: Oxford University Press,
Inc. pp. 306-319.
McManus, J.W., M.C.A. Ablan, S.G. Vergara, B.M. Vallejo,
L.A.B. Menez, K.P.K. Reyes, M.L.G. Gorospe and L. Halmarick, 1997. Reefbase
Aquanaut Survey Manual. ICLARM Educational Series. 18, 61p.
Sumich, J.L. 1996. An
Introduction to the Biology of Marine Life, sixth edition. Dubuque, IA: Wm.
C. Brown. pp. 255-269.
Turgeon, D.D. and R.G. Asch. In
Press. The State of Coral Reef Ecosystems of the United States and Pacific
Freely Associated States. Washington D.C.; NOAA.
Veron, JEN. 2000. Corals of the World. Vol 3. Australia: Australian Institute of Marine Sciences and CRR Qld Pty Ltd.
The following information on
La parguera, Puerto Rico is abridged from
La Parguera, Puerto Rico, USA,
Jeorge R. Garcia, Christoph Schmitt, Craig Herbere, and Amos Winter; United
Nations Educational, Scientific and
Cultural Organization; available
from : http://www.unesco.org/csi/pub/papers/garciab.htm, [Accessed: November 23,
2010].
|
Environment and development |
|
La Parguera, Puerto Rico, USA
Jorge R. García, Christoph Schmitt, Craig Heberer, and Amos Winter
Department of Marine Sciences, University of Puerto Rico, Isla Magüeyes Laboratories, La Parguera, PO Box 908, Lajas PR 00667 USA
The insular shelf of La Parguera, on the southwest
coast of Puerto Rico, is characterized by an extensive development of coral
reefs, seagrass beds, and mangrove forests. The dry, warm, and relatively stable
climate, low wave energy, high water transparency, relatively wide shelf,
oligotrophic offshore waters, and low urban coastal development are some of the
factors that contribute to the conditions of the marine ecosystem of La Parguera.
Interactions among coral reef, seagrass, and mangrove communities provide for a
highly productive, structurally complex, and biologically diverse ecosystem.
Coastal development and associated anthropogenic impact, technologically
advanced exploitation of fisheries, global climatic change, and natural events
all have potentially detrimental effects on marine ecosystems and need to be
analyzed from a regional perspective. We review and summarize information
leading to a baseline characterization of the ecosystem of La Parguera.
Introduction
La
Parguera is a coastal village within the township of
Lajas on the southwestern coast of Puerto Rico. Its insular shelf boundaries
extend from Punta Montalva in the east (66°59'W) to Punta Tocón in the west
(67°06'W) and from the coastline (18°01'N) to the shelf edge (18°07'N) (Fig. 1).
The southwestern coast is a generally dry and warm region, classified as a
subtropical dry forest life zone (Ewel and Whitmore, 1973). A chain of low
hills, known as Sierra Bermeja, separates the coastal plain from the Lajas
Valley. Sierra Bermeja acts as an important hydrographic boundary that confines
the watershed of La Parguera to the southern slopes of the Sierra and to the
relatively narrow coastal plain. The shelf is composed mainly of carbonates
deposited during the Cretaceous (Almy, 1965) and flooded some 5,000 to 9,000
years ago due to eustatic sea level rise (Goenaga, 1988), thereby forming the
neritic zone of La Parguera.
La Parguera
is recognized for the exceptional value of its marine resources, which include
two bioluminiscent bays (Bahía Fosforescente and Monsio José), a coastal
mangrove fringe with several small lagoons, mangrove islands associated with
coral reefs, seagrass beds, and perhaps the best developed, most extensive coral
reef ecosystem of the island. Such attributes, and the significant improvement
in transportation and infrastructure across the island, have transformed La
Parguera from a mostly undeveloped and quiet fishing village to a center of
tourism. Resorts, guest houses, and private vacation homes have proliferated
over the past ten years, and the transient population has increased at least
three-fold — from approximately 35,000 visitors per year (NOAA/DNR,
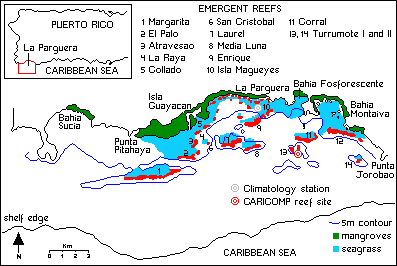
Fig. 1.
Location map of La
Paraguera, Puerto Rico, and its marine ecosystems.
1984) to more than 100,000. In order to halt chaotic
deforestation of the natural semi-arid forest and mangrove coastline, the Puerto
Rico Planning Board classified La Parguera as a Zone of Special Planning. In
further recognition of the ecological value of its marine resources, La Parguera
has also been designed as a Natural Reserve by the Department of Natural
Resources. At present, there is a proposal for the establishment of a Marine
Fishery Reserve at Turrumote Reef (Plan Development Team, 1990; García, 1990); a
previous effort to establish a Marine Sanctuary Program (NOAA/DNR, 1984) was not
accepted by the local community (Fiske, 1992). Field and laboratory research
facilities of the Department of Marine Sciences, University of Puerto Rico
Mayaguez Campus, are based on Magüeyes Island off La Parguera.
The coexistence and interdependence of coral reef,
seagrass, and mangrove communities within the insular shelf of La Parguera
result in a highly productive and structurally complex ecosystem with very high
biodiversity. Coral reefs act as barriers to wave action and permit the
establishment of seagrasses and fringing mangroves (Goenaga and Cintrón, 1979).
In turn, seagrasses and mangroves contribute organic matter for coral nutrition
and serve as important foraging and nursery habitats for coral reef fishes and
other organisms. Each of these communities can be regarded as highly productive
and taxonomically diverse. For example, mangrove lagoons function as nurseries
for many juvenile coral reef fishes (Austin, 1971;
Yáñez-Arancibia and Nugent, 1977;
Gonzalez-Sansón, 1983), many of which are
commercially important as adults (e.g., snappers, jacks, barracudas, and
others). The lagoons are also the natural habitat of resident populations of,
for example, snook, tarpon, ladyfish, mojarra, and sole that add to the
structural complexity and diversity of the ichthyofauna in La Parguera.
Likewise, seagrasses are particularly important foraging (transient) areas for
coral reef fishes and endangered species such as manatees and green sea turtles
(Gonzalez-Liboy, 1979) and, as well, provide a permanent niche for a highly
diverse and abundant flora (Glynn, 1964; Matthews, 1967) and fauna (Gonzalez-Liboy,
1979; Vicente, 1992).
Coral reefs extend throughout a wide range of depths and
distances from the coast in La Parguera and consequently are exposed to
gradients of physical, chemical, and biologically interacting forces (e.g.,
wave energy, light penetration, temperature, salinity, nutrient availability,
suspended sediments). These gradients affect the structure of the biological
community within reefs (e.g., vertical coral zonation patterns) and
between reefs (Morelock et al., 1977; Acevedo and Morelock, 1988). This
variability in community structure within and between reefs promotes the
biological diversity of coral reef-associated organisms. These changes in coral
reef community structure introduce variable patterns of sedimentation adjacent
to the reefs (Morelock et al., 1977), potentially influencing variability
in benthic communities associated with different sediment types. The submerged
shelf-edge reef of La Parguera is an important spawning site for coral reef
fishes (Colin and Clavijo, 1988) and serves as a foraging area for pelagic
(oceanic) predators. Such neritic-pelagic interaction contributes to
ichthyofaunal biodiversity and local fisheries production.
The insular shelf of La Parguera extends 8-10 km offshore; a
well developed coral reef formation exists at the border of the shelf (Morelock
et al., 1977) and serves as a first barrier against wave action. Two
other lines of barrier reefs provide further protection for the mangrove
coastline and submerged seagrass beds of La Parguera. Nevertheless,
storm-generated waves may play an important role in the distribution, structural
complexity, and biodiversity of local coral reefs and associated communities
(Yoshioka and Yoshioka, 1989).
Climate and
Oceanography
La Parguera is located on the southwestern coast of Puerto
Rico in the subtropical climate belt influenced by easterly trade winds during
90% of the year. However, by the time the moisture-laden trade winds have
crossed the island and reached La Parguera, most of the moisture has been lost.
Therefore, La Parguera is one of the driest and hottest areas along the coast of
Puerto Rico; the average annual rainfall 1961-1990 was 74.52 cm (Table 1),
compared to 132.74 cm at San Juan. The "rainy season" occurs during the fall
(average 35.61 cm), the "dry season" occurs in winter (average 9.12 cm). The
highest one-day rainfall 1961-1990 was 35.31 cm on September 17, 1975 (Table 1).
Most of the high rainfall amounts are caused by tropical storms that stall in
the northeastern Caribbean. Occasional cold fronts in winter, which may
sometimes be associated with large amounts of rain in Puerto Rico, seem not to
affect the southwestern corner of the island. Total precipitation amounts vary
from year to year. The lowest annual rainfall 1960-1991 was 40.94 cm in 1977,
the highest was 123.57 cm in 1960.
Coral Reefs
According to Almy (1969), coral reefs in La Parguera
originated from erosion and deformation of Upper Cretaceous limestones (with
interbedded mudstones and volcanic rocks) into a WNW-ESE trending syncline. The
northern limb of the syncline is the Sierra Bermeja, and the southern limb is a
platform of lower relief represented by the coral reefs on the shelf. The rise
in sea level associated with the last Pleistocene glaciation (Wisconsin) flooded
the lower limestone ridges on the shelf, providing appropriate sites for coral
growth and subsequent reef development (Glynn, 1973; Goenaga and Cintrón, 1979
Substrate, depth, and water transparency conditions in La Parguera allowed for
extensive development of coral reefs during the mid-Holocene (Vicente, 1993).
Two
distinct lines of emergent reefs align east-west, parallel to the coastline, and
divide the insular shelf of La Parguera into inner, middle, and outer shelf
zones (Morelock et al., 1977). There are many other smaller submerged
patch reefs dispersed throughout the shelf, as well as a large submerged reef at
the shelf edge. Altogether, it has been estimated that coral reefs occupy about
20% of the La Parguera insular shelf (Morelock et al., 1977). Margarita
Reef, the westernmost in the second line of emergent reefs, is the largest of
the "island reefs," with a maximum underwater extension of 4.2 km. The
shelf-edge reef is located at 20 m and has a "buttressed" appearance, with
channels cut into the slope down to 30 m (Morelock et al., 1977).
|
Table 1.
Historical monthly mean rainfall record from the Isla Magüeyes
NOAA 665693). |
|||||||
|
Total Rainfall |
|||||||
|
|
Mean |
High |
Low |
1-Day Max. |
|||
|
|
cm |
cm |
year |
cm |
year |
cm |
dd/yyyy |
|
January |
2.77 |
7.87 |
1984 |
0.00 |
1967 |
5.46 |
27/1973 |
|
February |
2.41 |
11.07 |
1984 |
0.10 |
1975 |
6.60 |
04/1984 |
|
March |
2.69 |
9.88 |
1983 |
0.30 |
1964 |
7.16 |
13/1983 |
|
April |
3.28 |
10.52 |
1983 |
0.13 |
1974 |
5.46 |
21/1983 |
|
May |
6.73 |
29.29 |
1986 |
0.00 |
1974 |
14.63 |
28/1980 |
|
June |
3.76 |
20.80 |
1987 |
0.51 |
1977 |
11.94 |
15/1990 |
|
July |
4.45 |
19.96 |
1984 |
0.05 |
1976 |
18.24 |
05/1984 |
|
August |
8.64 |
32.82 |
1978 |
1.50 |
1972 |
29.85 |
17/1978 |
|
September |
11.79 |
39.34 |
1975 |
2.84 |
1971 |
35.31 |
17/1975 |
|
October |
12.93 |
54.69 |
1985 |
2.08 |
1965 |
26.04 |
07/1985 |
|
November |
10.87 |
41.40 |
1987 |
0.00 |
1962 |
18.54 |
04/1984 |
|
December |
3.94 |
16.21 |
1981 |
0.00 |
1979 |
9.53 |
11/1981 |
|
|
|
|
|
|
|
cm |
dd/mm/yyyy |
|
Annual |
74.52 |
110.90 |
1978 |
40.94 |
1977 |
35.31 |
17/09/1975 |
|
Winter |
9.12 |
29.16 |
1961 |
2.44 |
1990 |
9.53 |
11/12/1981 |
|
Spring |
12.70 |
34.24 |
1986 |
3.23 |
1974 |
14.63 |
28/05/1980 |
|
Summer |
16.84 |
35.79 |
1988 |
5.11 |
1967 |
29.85 |
17/08/1978 |
|
Autumn |
35.61 |
79.12 |
1985 |
9.83 |
1980 |
35.31 |
17/09/1975 |
Mangroves
Some of the emergent portions of the shelf reefs at La
Parguera are colonized by mangroves. The degree of exposure to the incoming
waves limits mangrove development on these offshore islands (Yoshioka, 1975).
Red mangrove, Rhizophora mangle, is the dominant species on island reefs;
a few white mangroves (Laguncularia racemosa) are also present. Mangrove
development is greatest in zones of intermediate wave energy. On the exposed
outer cays, the strong surf does not allow deposition of the fine sediments
needed for the growth of red mangroves. On the middle shelf zone, waves and
currents are strong enough to maintain a constant flow of water, yet allow for
accumulation of fine sediments. Consequently, red mangroves prevail at these
middle shelf reefs. The inner shelf reefs are not subject to enough wave energy
to maintain adequate flushing; consequently, these reefs normally have strong
transverse salinity gradients. Salt builds up in the center of these islands and
enables the succession of red mangroves by the more salt-tolerant black mangrove
(Avicenna). Prolonged accumulation of salt eventually leads to the death
of the black mangroves.
Seagrass
Beds
The extensive seagrass beds that occur in southwestern
Puerto Rico, in close proximity to some of the island’s most pristine coral reef
and mangrove habitats, provide nursery and feeding grounds. In addition to
providing basic nutrients, primary productivity, and stable habitats, these beds
provide essential foraging grounds for such endangered marine species as the
West Indian manatee, Trichechus manatus, and the green sea turtle,
Chelonia mydas.
Thalassia testudinum,
Syringodium filiforme, Halophila decipiens, and Halodule wrightii
inhabit the insular shelf zones on both the Atlantic and Caribbean coasts of
Puerto Rico as well as the nearby islands of Vieques and Culebra.
Large seagrass beds are established in the La Parguera area,
with Thalassia and Syringodium being the most abundant and widely
distributed seagrasses over the insular shelf and also in the back-reef zones of
middle shelf reefs. The most extensive seagrass beds are found within the 2-m
depth contour, fringing the red mangrove coastline. Mangrove forests border
almost the entire southwestern coastline, and mangrove islets are common inside
the inner shelf (Cintrón et al., 1978)
The seagrass beds of southwestern Puerto Rico appear to be in
good condition and serve as a key component, intimately and functionally
associated with the coral reef and mangrove ecosystems, in providing important
nursery and foraging grounds for many commercially important fish and
invertebrate populations. In order to ensure the long-term health and
sustainability of these nearshore marine ecosystems, future development plans
will have to take into consideration the physical and biological requirements of
the seagrass beds and offer protection whenever possible, as well as restoring
the original mangrove fringe where it has been cut or destroyed.
References
Acevedo,
R., J. Morelock. 1988. Effects of terrigenous sediment influx on coral reef
zonation in southwestern Puerto Rico. Proceedings of the 6th International
Coral Reef Symposium, Australia, 2:189-194.
Almy,
C. 1965. Parguera Limestone, Upper Cretaceous Mayaguez Group, Southwestern
Puerto Rico. Ph.D. Thesis, Rice University, Houston TX, USA.
Austin,
H. M. 1971. A survey of the ichthyofauna of the mangroves of western Puerto Rico
during December 1967-August 1968. Caribbean Journal of Science, 11
(3-4):171-178.
Cintrón,
G., A. Jugo, D. Pool, G. Morris. 1978. Mangroves of arid environments in Puerto
Rico and adjacent islands. Biotropica, 10(2):23-35.
Colin,
P. L., I. E. Clavijo.
1988. Spawning activity of fishes producing pelagic eggs on a shelf edge coral
reef, southwestern Puerto Rico. Bulletin of Marine Science, 43:249-
279.
Ewel, J. J., L. Whitmore. 1973.
The Ecological Life Zones of Puerto Rico and the U.S. Virgin Islands.
Forest Service Research Paper ITF 18, U.S. Department of Agriculture, 18 pp.
Fiske,
S. J. 1992. Sociocultural aspects of establishing marine protected areas.
Ocean and Coastal Management, 18:25-46.
García,
J. R. 1990. Population dynamics and production of Phyllorhiza punctata (Cnidaria:Scyphozoa)
in Laguna Joyuda, Puerto Rico. Marine Ecology Progress Series, 64:243-251.
Glynn,
P. W. 1964. Common Marine Invertebrate Animals of the Shallow Waters of
Puerto Rico.
Instituto de Cultura Puertorriqueño, Historia Natural de Puerto Rico, San Juan
PR, USA, 48 pp.
Goenaga,
C., G. Cintrón. 1979. Inventory of the Puerto Rican Coral Reefs.
Department of Natural Resources, Coastal Zone Management Program, San Juan PR,
USA, 190 pp.
Gonzalez-Liboy,
J.
1979. An Examination of the Present Condition of Seagrass Meadows in La
Parguera, Puerto Rico. Final Report, State of Puerto Rico Project No. 4,
June, 1979.
Gonzalez-Sansón,
G. 1983. Estructura de las comunidades de peces en las lagunas costeras de Tunas
de Zaza, Cuba. Revista de Investigaciones Marinas, 4:135-158.
Matthews,
B. 1967. An Ecological Guide to the Littoral Fauna and Flora of Puerto Rico.
Department of Education, San Juan PR, USA, 37 pp.
Morelock,
J., N. Schneiderman, W. Bryant. 1977. Shelf reefs, southwestern Puerto Rico.
Studies in Geology,
4:17-25
NOAA/DNR.
1984. Declaración de Impacto Ambiental Final y Plan de Manejo Para el
Sanctuario Marino Nacional de La Parguera.
National Oceanic and Atmospheric
Administration/Puerto Rico Department of Natural Resources, 277 pp.
Plan Development Team.
1990. The Potential of Marine Fishery Reserves for Reef Fish Management in
the U. S. Southern Atlantic. NOAA Technical Memorandum NMFS- SEFC-261, 40
pp.
Vicente,
V. P. 1992. A summary of ecological information on the seagrass beds of Puerto
Rico. In: Coastal Plant Communities of Latin America, pp 123-133.
Academic Press, New York, NY, USA.
Yoshioka,
P. 1975. Mangrove root communities in Jobos Bay.
In:
Puerto Rico Nuclear Center: Aguirre Environmental Studies, Jobos Bay, Puerto
Rico, Final Report, 1975,
1:50-65.
Yáñez-Arancibia,
A., R. S. Nugent. 1977. El papel ecológico de los peces en estuarios y lagunas
costeras. UNAM Anales
Centro Ciencias del Mar y Limnología,
4:107-114.
The following information on
Puerto Rico is abridged from Chapter
16 of Status of Coral Reefs of the World:
2008,Wilkinson, C, ed; Global Coral Reef Monitoring Network and Reef and
Rainforest Research Center, Townsville, Australia; available
from: http://www.reefbase.org [Accesse:
November 23, 2010]. Information/data/maps/provide by REEFBase
(http:www.reefbase.org)
Editors: Mark E. Monaco, Jeannette
Waddell, Alicia
Clarke,
Chris
Caldow,
Christopher
F.G. Jeffrey, Simon Pittman
Introduction
This chapter covers coral reef areas under the
jurisdiction of the USA in the Wider Caribbean: Florida; Flower Garden Banks;
Puerto Rico; U.S. Virgin Islands; and Navassa. The following information is
condensed from six chapters of The State of Coral
Reef Ecosystems of the United States and Pacific Freely Associated States: 2008.
Access to the full text of this comprehensive report is available at:
http://ccma.nos.noaa.gov/stateofthereefs.
Status
of the
Coral
Reefs In
2008
Puerto Rico:
Coral cover has been variable spatially, temporally and between studies over the
years, but all studies report a general decline from bleaching and disease, as
well as sediment and nutrient inputs. Up to 97% of corals bleached at monitoring
sites with about 50% coral
229
Status of the Coral Reef Ecosystems in the U.S. Caribbean, and Gulf of Mexico
mortality during the 2005 bleaching event; a massive white plague-like outbreak
followed that resulted in 20 - 60% decline in coral cover on the east coast
within 6 months. In addition, coral cover in southwest Puerto Rico is inversely
correlated with increased turbidity from sediment and nutrient inputs.
Status
of
Coral
Reef
Fishes
and Invertebrates
Puerto Rico:
Shallow water reef fish abundance has generally declined;
for example Nassau and goliath groupers (E.
striatus and E.
itajara) and queen conch (Strombus
gigas) are being over-fished, as well as snapper
and parrotfish. Fish spawning aggregations have also declined, especially for
the larger, more commercially desirable species. Models developed by the
University of Miami showed the majority of species are over-fished, with some
substantially over-fished. The only large groupers that remain are known to
cause ciguatera poisoning in humans. Although there has been a substantial
decrease in fishing effort over the last 20 years, there is still an excess of
fishing pressure. There has been a shift in community structure of fished
groupers in southwest Puerto Rico from 2001-2006. Initially red hind (E.
guttatus) were the most prevalent species, then the
smaller Coney (Cephalopholis fulvus)
became more prevalent, and most recently the smallest grouper (Graysby,
Cephalopholis cruentatus)
constituted well over 50% of the fished groupers in 2003, and more than 90% in
2006.
Anthropogenic
Threats to Coral Reefs
The top 5 threats to US Caribbean coral reefs include: elevated sea water
temperature resulting in coral bleaching; coral diseases; tropical storms and
hurricanes; unsustainable coastal development resulting in increased sediment
and nutrient runoff; and over-fishing and damage from fishing. However the
significance of each threat varies considerably. For example Florida, Puerto
Rico and the USVI are heavily populated, unlike the Flower Garden Banks and
Navassa.
The most common diseases
affecting Puerto Rican corals were white plague-II, yellow band, white band,
black band, aspergillosis and coralline white band; but the distribution and
prevalence was highly variable. Frequent epizootic events result in significant
losses of coral cover on most reefs around the island, particularly during the
summer; but bleaching and disease disappear when temperatures drop in winter.
Reporting on reef fisheries status remains a major challenge for resource
managers, largely due to inadequate data on commercial and recreational
fisheries. Managers report that
233
Status of the Coral Reef Ecosystems in the U.S. Caribbean, and Gulf of Mexico
Current Conservation
Management
Activities
U.S. Virgin Islands and Puerto Rico: Since the early 1960s, many MPAs have been established in the USVI by Federal and Territorial agencies, including the recently enlarged Virgin Islands Marine National Monuments, Marine National Parks, a Marine Conservation District (MCD), several small marine reserves, Spawning Aggregation Areas (SPAGs) and 18 Areas of Particular Concern. On St. Croix, the East End Marine Park was established in 2003. Existing MPAs vary greatly in size, location and purpose and represent a wide regulatory spectrum, ranging from very little regulation (multi-use areas) to the total exclusion of extractive activities (marine reserves). The few studies inside and outside MPAs in the U.S. Caribbean have highlighted problems related to suboptimal boundary delineations, high human impacts, low resilience to disturbance and limited recovery in marine reserves. The Hind Bank Marine Conservation District south of St. Thomas was established in 1999 and became the first no-take federal 235 Status of the Coral Reef Ecosystems in the U.S. Caribbean, and Gulf of Mexico.